 Electrochemical activation (ECAS) is a technology which produces activated solutions (including water) through electrochemical influence. Activated solutions can be used in different areas of human activity, as far as they maintain heightened electrochemical activity. The time of this activity is limited: it differs from several hours to several months.
Electrochemical activation (ECAS) is a technology which produces activated solutions (including water) through electrochemical influence. Activated solutions can be used in different areas of human activity, as far as they maintain heightened electrochemical activity. The time of this activity is limited: it differs from several hours to several months.
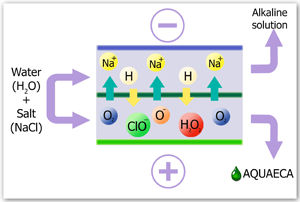
As a result of electrochemical activation, water turns into a metastable (activated) state, meanwhile showing enhanced reactionary capacity in different physical and chemical processes. The water that is activated at an cathode (catholyte) is notable for improved electron activity and has marked properties of a deoxidizer. Accordingly, the water, which is treated at an anode (aquaeca) is characterized by reduced activity of electrons and shows the properties of oxidizer.
There are two general areas where ECAS can be applied: synthesis of safe disinfection solutions and production of activated water, which is also called Emerald water Emerald water, which is produced by the joint influence of anode and cathode, combines some properties of catholyte and aquaeca, yet also has another peculiarity: as the water molecules’ structure restoration, and redox potential lowering. In addition to that, some Izumrud models can produce. Dead and Living water.
In disinfection, ECAS is used to produce high-performance ecological solutions in different fields of human activity. In almost every area, where there is any contact with fluid, ECAS technologies are applicable. Unlike most of electrochemical processes, source substances in ECAS processes are diluted water-saline solutions, fresh or low-mineralized water, i.e. fluids with low electro conductivity. The final products of ECAS are not concentrated chemical substances, but activated solutions, i.e. low-mineralized fluids in activated state. That’s why ECAS technologies are safe to apply both at home and in industry.
